
Quality and Certificates
This has been our constant aim ever since we began over 65 years ago. Today this objective remains a guiding star for our team of over 55 professionals, who ensure that the end product complies with all national and EU standards.
The selection of materials and the constant effort to improve in terms of both processes and formulations, and in the incorporation of new technologies, has enabled us to create products with exceptional characteristics that set them apart from the rest.
ANSI/ADA Standard No. 15 and DIN EN13914 quality standards.
ISO/TR 7405 Biocompatibility.
ISO 22112:2005 Standard specific for synthetic polymer and ceramic teeth.
ISO 13485:2016 Standard for the design and production of medical devices.
EC production quality assurance (MDD 93/42/EEC) for the production of medical devices.

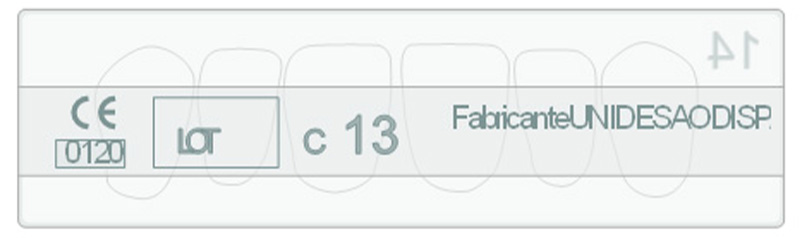
Traceability
Each set has its own ID, which guarantees the traceability of all our teeth.
One of the keys to Unidesa-Odi’s success is the implementation of its own traceability and monitoring systems, which enable the company to closely control each set (made up of one card – minimum unit of sale) that is produced and delivered.
All the systems guarantee compliance with the strictest standards set by European directives and the FDA.
A code printed in relief on the rear of the splint makes each one of our cards a unique item. This code indicates the batch number and the registration number and proves that the card is of European origin. In this way the identity of each card is guaranteed to a level that very few manufacturers can offer.



